Which Of The Following Statements Best Describes The Quantum Property Spin?
Which of the following statements best describes the quantum property spin?. Which of the following statements best describes the quantum property spin. The two values of the spin quantum number allow each orbital to hold two electrons. Which Of The Following Statements About Quantum Numbers Is TRUE.
Spin is not meant to be taken literally but measures the inherent angular momentum of a subatomic particle. Which of the following statements best describes the quantum property spin. C Spin is a measure of the rotation rate of a subatomic particle.
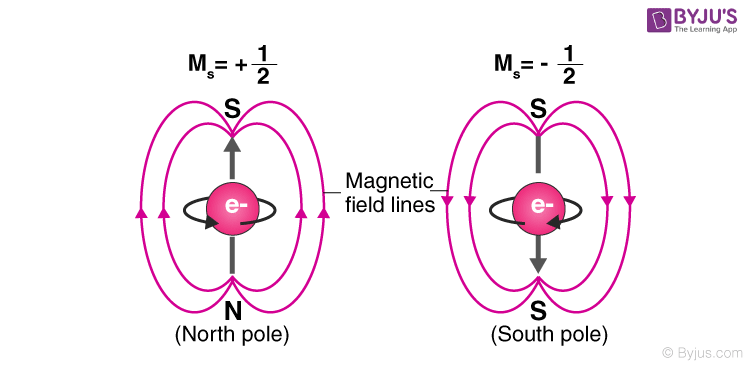
If those three numbers are identical for two electrons the spin numbers must be different in order for the two electrons to be differentiated from one another. Preview this quiz on Quizizz. Spin Quantum Number m s The spin quantum number describes the spin for a given electron.
The magnetic quantum number relates the spatial orientation of the electron orbital. ASpin is a measure of the rotation rate of a subatomic particle. 98 A Spin is not meant to be taken literally but measures the inherent angular momentum of a subatomic particle.
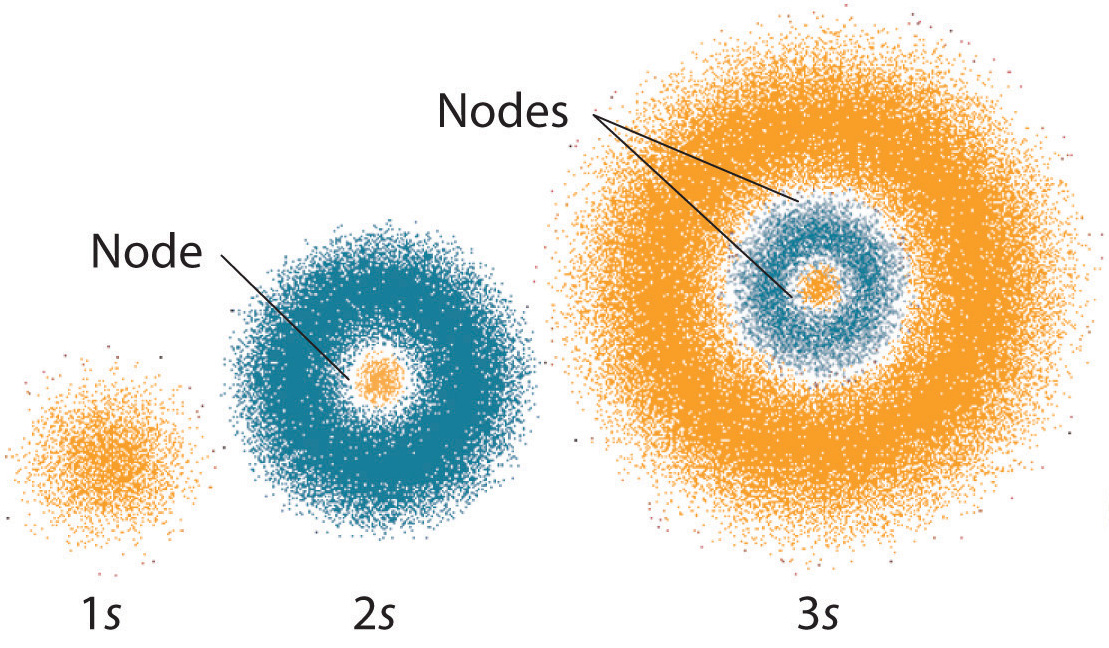
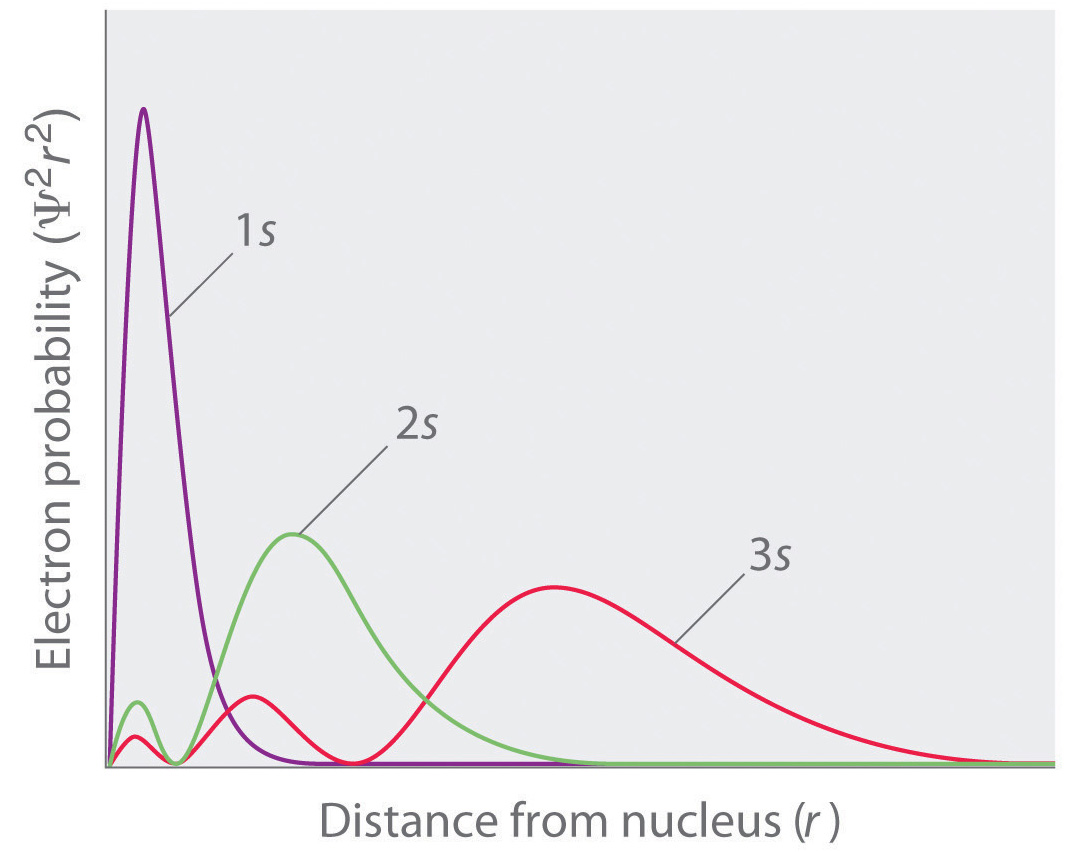
Table above shows the possible magnetic quantum number values m 1 for the corresponding angular momentum quantum numbers l of l 0 l 1 l 2 and l 3. The Principle Quantum Number Describes The Size Of An Orbital E. The spin quantum number is not the outcome of the Schrodinger equation.
Spin is not meant to be taken literally but measures the inherent angular momentum of a subatomic particle. The Spin Quantum Number Describes The Orientation Of The Orbital B. B Spin is a property that applies only to large objects like baseballs.
ASpin is a property that applies only to large objects like baseballs. The electron may be in.
Spin is a fundamental quantum property of subatomic particles and does not result from their physical rotation.
ASpin is a property that applies only to large objects like baseballs. CSpin is a property that applies only to large objectslike baseballs. Which of the following statements best describes the quantum property spin. Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one of the electrons that can occupy the orbital. C Spin is a measure of the rotation rate of a subatomic particle. The Spin Quantum Number Describes The Orientation Of The Orbital B. The magnetic quantum number describes the. Which Of The Following Statements About Quantum Numbers Is TRUE. BSpin is not a fundamental property but rather something that can change randomly at any time.
The electron may be in. B Spin is a property that applies only to large objects like baseballs. Which of the following statements is not. The formulation of the quantum world that most folks are familiar with say the famous Schrodinger wave equation the allows us to compute probabilities of particle locations. In quantum field theory in the case of a massive field the Casimir invariant WμWμ describes the total spin of the particle with eigenvalues displaystyle W 2W_ mu W mu -m 2s s1 where s is the spin quantum number of the particle and m is its rest mass. The Spin Quantum Number Describes The Orientation Of The Orbital B. An electron can have one of two associated spins latexdisplaystyleleftfrac12rightlatex spin or latexdisplaystyleleft.






























Post a Comment for "Which Of The Following Statements Best Describes The Quantum Property Spin?"